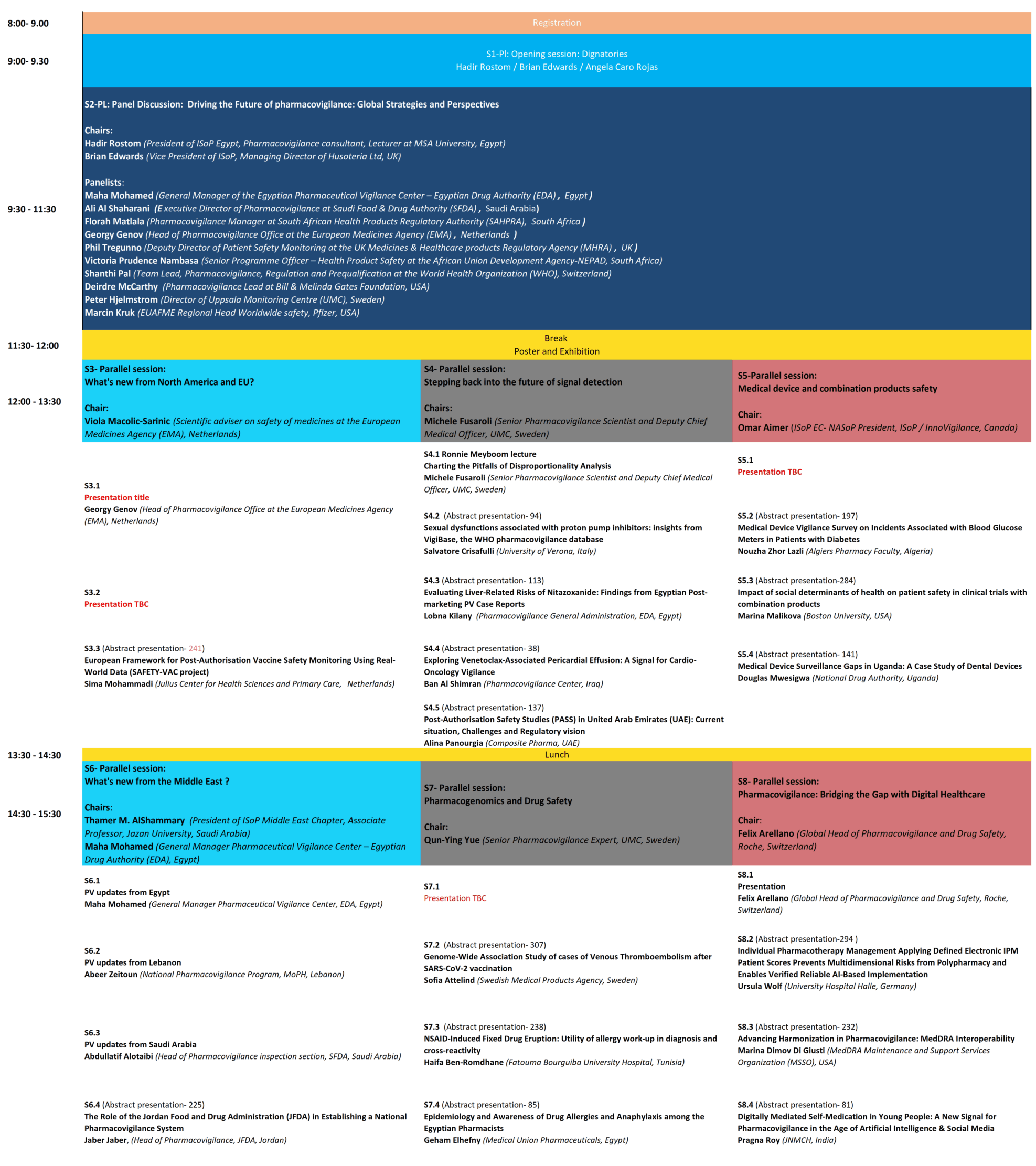
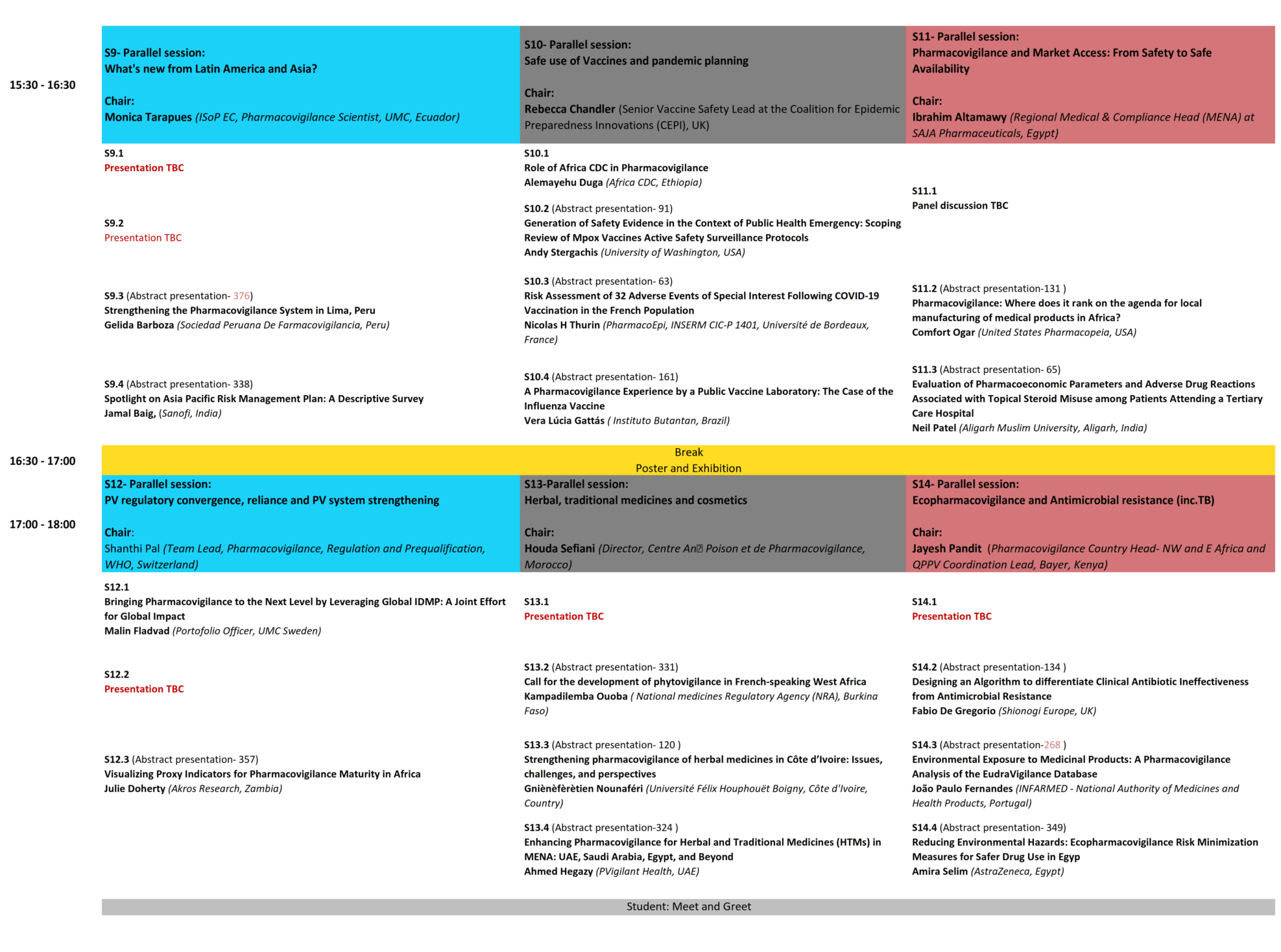
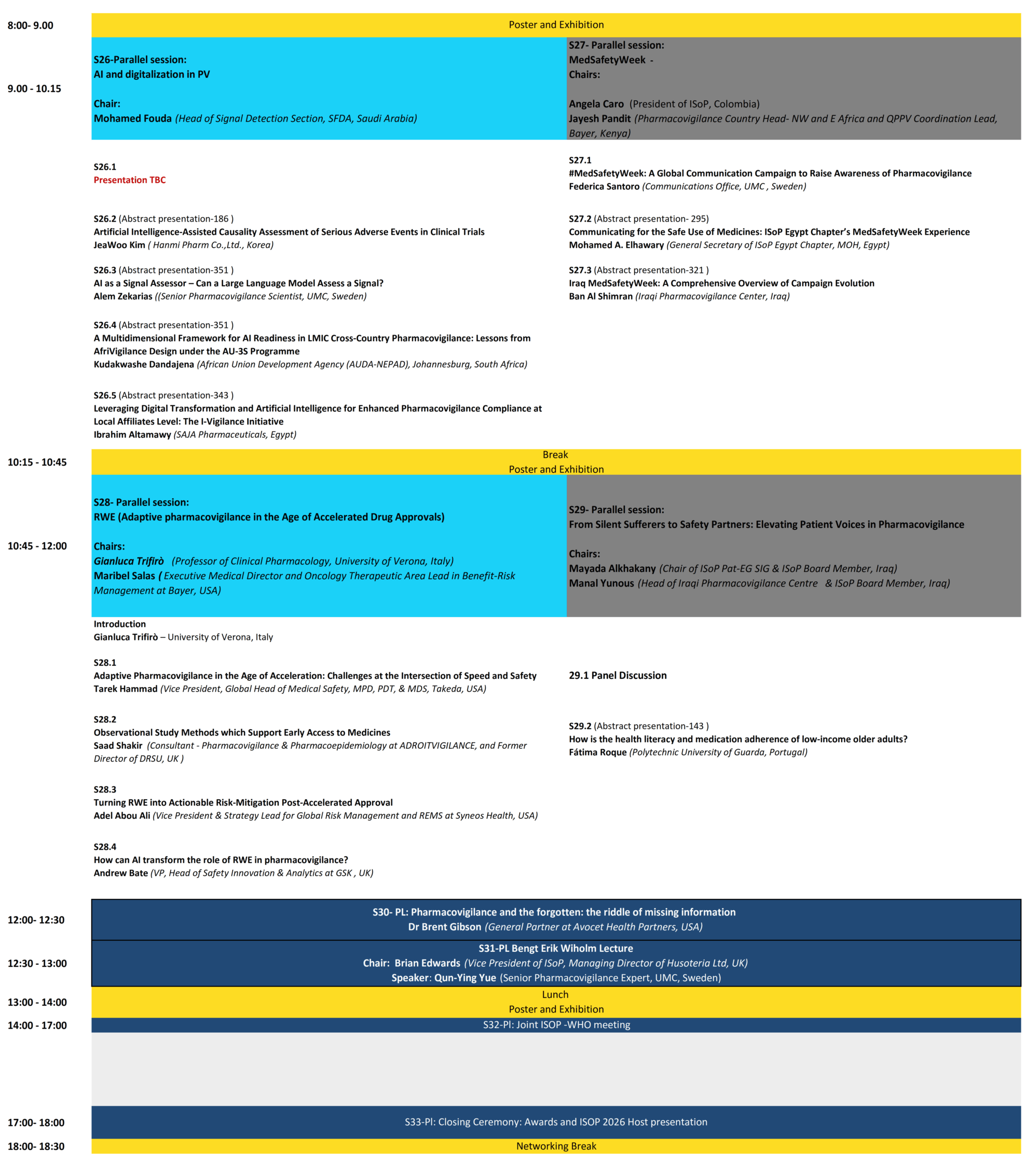
Programme
Scientific Program
All Pre-conference training courses and the conference are in the same venue; InterContinental Cairo Semiramis
Parallel training courses
Course 1:
Pharmacovigilance in the Community and Healthcare
Explores pharmacovigilance in healthcare settings and role of healthcare professionals.
Ideal for enhancing clinical practice knowledge.
Course Coordinator:
Alem Zekarias (UMC, Sweden)
Course 2:
Advanced Course in Pharmacovigilance for the Industry
Aimed at industry PV professionals. Explores how to advance PV practice with the rapidly changing requirements.
Perfect for advancing industry expertise.
Course Coordinators:
Mayada Alkhakany (Chair of ISoP Pat-EG SIG & ISoP Board Member, Iraq)
Santiago Schiaffino (Senior Director, Patient Safety Biopharma at AstraZeneca, Spain)
Course 3:
Challenges and strategies for strengthening Pharmacovigilance of Vaccines
Essential for Pharmacovigilance centers, Immunization Programs and public health.
Course Coordinator:
Ghita Benabdallah (ISoP Board Member, National Pharmacovigilance Center, Morocco)
Course 4 &5:
Pharmacoepidemiology and Benefit/Risk Assessment
Essential for Industry, regulators and public health
Course 4 Coordinator:
Gianluca Trifirò (, Professor of Clinical Pharmacology, University of Verona, Italy)
Course 5 Coordinator
Adel Abou Ali (Vice President & Strategy Lead for Global Risk Management and REMS at Syneos Health, USA)
Course 1
Pharmacovigilance in the community and healthcare-
8:30- 9:00 Registration
-
9:00- 10:00 Role of HCPs in the PV
-
10:00- 11:00 Human factors and safety of medicines and devices
-
11:00- 11:30 Break
-
11:30 - 12:30 Medication errors and Medication Safety Officer
-
12:30- 13:30 Spontaneous reporting
-
13:30- 14:30 Lunch
-
14:30- 15:30 Risk Management
-
15:30- 16:30 Real world evidence, healthcare research and epidemiology with the PV community
-
16:30- 16:45 Break
-
16:45 - 17:30 Plenary session: What is the way forward?
Course 2
Advanced pharmacovigilance for the industry: Mastering Inspections, Data Quality & Risk Management in the Digital Age-
8:30 -9:00 Registration
-
9:00- 10:00 Inspection vs. Auditing: Strengthening PV Compliance
-
10:00 - 11:00 Quality of Safety Data: New Technologies for Enhancement
-
11:00- 11:30 Break
-
11:30- 12:30 Medical Literature Review & Its Impact on Signal Detection
-
12:30- 13:30 Risk Minimization Measures: From Challenge to Opportunity
-
13:30- 14:30 Lunch
-
14:30- 15:30 Crisis Management in Pharmacovigilance
-
15:30- 16:30 Future of Pharmacovigilance: Leveraging Innovation & Digital Transformation
-
16:30- 16:45 Break
-
16:45- 17:30 | Interactive Case Study & Q&A
Course 3
Challenges and strategies for strengthening Pharmacovigilance of Vaccines-
8:30 -9:00 Registration
-
Introduction to pharmacovigilance of vaccines
-
11:00- 11:30 Break
-
Adverse events following immunisation
-
13:30- 14:30 Lunch
-
Methods of analysis
-
16:30- 16:45 Break
-
Strategies and prioritises for strengthening pharmacovigilance of vaccines systems
Course 4
Introduction to pharmacoepidemiology-
8:30 -9:00 Registration
-
9:00- 9:45 Principles for Observational Real-World Studies including their Strengths and Weaknesses
-
9:45 - 10:30 Data sources and study design
-
10:30- 11:00 Sources of bias and confounding
-
11:00- 11:30 Break
-
11:30- 12:15 Continue Sources of bias and confounding
-
12:15- 13:00 Role of Observational Data in Regulatory Decision Making: The Drug Safety Paradigm
18:30- 20:30 Welcome Reception

19:30- 20:30 Transportation from the conference venue to the dinner
20:30- 23:30 Conference Dinner
23:30 – 24:00 Transportation back to the conference venue
Conference Dinner
Join us for that special dinner of ISoP 2025 Cairo.
Venue: Pyramids, Giza
Ticket sold at 50 USD
Dress code: Smart casual
Transfer to and from dinner venue will be provided.
Purchasing tickets is available for ISoP 2025 Cairo participants and accompanying persons here
For inquires contact us at isop2025cairo@falconfenix.com